Welcome to the Research Group of Professor Mike Hannon
| Home | Research | Group Members | Publications | Collaborations | Joining the Group | Photos | Contact Us | Location | Links |
 |
Welcome to the Research Group of Professor Mike Hannon |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||
| Press release: DNA Recognition | DNA Recognition | Cellular and Nuclear Targeting | Supramolecular Architectures |
We have developed a synthetic approach to supramolecular architectures based on an imine-based strategy. The elegance of this strategy is the ease-of-synthesis which allows multigramme quantities of sophisticated, defined molecular architectures to be prepared rapidly in one-pot reactions from commercial amines and aldehydes. This allows the properties to be studied and the architectures to be systematically varied and systematically functionalised. A variety of supramolecular architectures such as double and triple helices, triangles and grids can be prepared. The architecture adopted is controlled by the selection of the metal and the design of the ligand. The molecular design can also be used to encode the precise micro-architecture and we have explored routes to encode additional information into the array such as strand directionality, groove size and chirality. This ability to control architecture at the molecular level is important as it offers a potential route to systematically encode the properties of a molecule or material. This programme underpins and enables the DNA recognition programme detailed in 1.


Two enantiomers of a trinuclear circular-helical cation
For examples of our work in this area see
Chem. -Eur. J., 2007, 13, 9286-9296
Angew. Chem. Int. Ed., 1999, 38, 1277-1278
Chem. Eur. J., 2002, 8, 4957-4964
Review of our work and that of others on helicates: Supramolecular Chem, 2004, 16, 7-22.
Discrete architectures are attractive targets, but libraries of architectures are also of considerable interest, since the library equilibria can be responsive to external agents (such as anions, solvents, guests or temperature) imparting molecular level sensing or switching functions. We have introduced the concept of chemical "frustration" (from multiple competing interactions present in the system) as a method to design libraries of these supramolecular architectures.

For examples of our work in this area see
Chem. Eur. J., 2002, 8, 2225-2238
Dalton Trans., 2003, 2141-2148
Chem. Eur. J., 2007, 13, 9286-9296
Covalent ligand synthesis introduces limitations to the size of the supramolecular array. Our approach to circumvent this is to use multiple recognition events in sequence, i.e. to use an initial supramolecular event to construct small supramolecular units which are then used as building blocks in a second supramolecular event to give a higher order (and larger) aggregate. We have illustrated how metal-ligand and π-π interactions can be used in concert and demonstrated that H-bond aggregation may also be used. The molecular shape or architecture can be used to control the aggregation of metallo-supramolecular units into larger arrays. Thus arc-shaped units can be used to generate two-dimensional cyclic arrays and bowl-shaped units generate three-dimensional closed polyhedra of nanoscale dimensions.

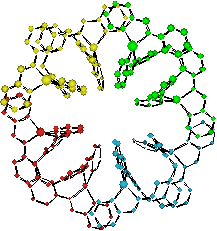
Examples of supramolecular aggregates
For examples of our work in this area see
Angew. Chem. Int. Ed., 2002, 41, 4244-4247
Angew. Chem. Int. Ed., 2001, 40, 1078-1079
Dalton Trans., 2006, 22, 2635-2642
Chem. Eur. J., 2004, 10, 5737-5750
We have used this sequential assembly approach to design red and blue luminescent solid state supramolecular arrays. By using a layer-by-layer electrostatic deposition approach, multilayers of these luminescent arrays may be assembled on solid supports by simple solution dipping techniques. This provides a simple route for device incorporation. We have demonstrated that the structure of the materials is the same in the layers as in the solid state allowing us to move from 'crystal engineering' to processable materials. Work is continuing in this area and will be extended to assembly of photo- and electro-active metallo-units into nanoscale supramolecular wires in collaboration with Pikramenou at Birmingham and others.
For examples of our work in this area see
Angew. Chem. Int. Ed., 2001, 40, 3862-3865
J. Chem.Soc., Dalton Trans., 2000, 1447-1461
Dalton. Trans., 2006, 24, 3025-3034

Schematic illustrating multilayer immobilisation
A further approach that we are exploring to bridge the gap between supramolecular architecture and materials science is the incorporation of the supramolecular species into polymers. We have prepared through monomer-functionalised supramolecular architectures and co-polymerised with co-monomers to give polymers containing supramolecular cross-links. The goal is to use the well-defined supramolecular architecture to influence the polymer architecture and thus the material properties.
For examples of our work in this area see
Angew. Chem. Int. Ed., 2001, 40, 1081-1084
Organogelators: , J. Mater. Chem., 2008, 18, 489-494