Welcome to the Research Group of Professor Mike Hannon
| Home | Research | Group Members | Publications | Collaborations | Joining the Group | Photos | Contact Us | Location | Links |
 |
Welcome to the Research Group of Professor Mike Hannon |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||
| Press release: DNA Recognition | DNA Recognition | Cellular and Nuclear Targeting | Supramolecular Architectures |
University of Birmingham press release for International science journals and magazines
The First Completely New Way Of Recognising DNA For 40 Years!
Scientists led by Mike Hannon at the University of Birmingham and Miquel Coll at the Spanish Research Council in Barcelona have discovered a new way that drugs can bind to DNA. The work is reported in the flagship chemistry journal Angewandte Chemie (2006 volume 45 issue 8 pages 1227-31) who have classed the work as a VIP paper and have chosen to feature it on the front cover of 2006 issue 8.

A new mode of DNA binding; recognition of the centre of the 3-way DNA junction
DNA contains the information which encodes life itself; its double-helical structure was recognised 50 years ago by Watson and Crick (1953) and the structure is now termed B-DNA. DNA holds the human genetic code. Scientists soon started designing drugs to target DNA and have used them to treat diseases such as cancer, viral infections and sleeping sickness.
To express the information contained in the DNA, the DNA is copied into multiple copies of a similar molecule RNA, each copy of which which holds exactly the same information that was in the original copy. The RNA molecules are then used to create proteins (with a further amplification in the overall number of copies!) which have a specific job to carry out: For example, they can be enzymes. To combat disease drug targets can be developed to work with DNA, RNA or proteins. For scientists, it is easier to target DNA, as (in principle) only one molecule of the drug target is needed. In the 1960s, scientists discovered three principal ways in which drug molecules could bind to DNA.
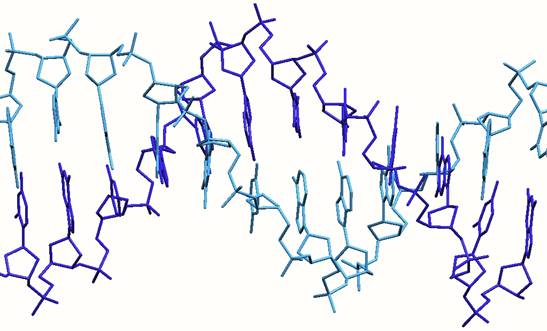
Intercalation. The first binding mode involves flat or planar aromatic molecules slotting in between the DNA base pairs at the heart of the DNA. This is termed “intercalation”. An early example of such a drug used to treat cancer was doxorubicin (trade name ‘Adriamycin’ or ‘Rubex’). This drug, launched in the 1960s, gave much impetus to the field and has been followed by a number of variants. Doxorubicin and its analogues are classified by clinicians as an "anthracycline antiobiotics."

Structure of a DNA oligonucleotide with two doxorubicin drugs (shown in pink) intercalated between the base pairs
Groove binding. This mode of binding involves molecules sitting in the grooves which are formed as the DNA strands wrap about each other to form the spiral (double-helical) structure. Most small molecule drugs fit snugly in the minor groove. Examples of such drugs include berenil and stilbamidine, pentamidine. While this mode of binding was recognized by the late 1960s, some of the drugs were already in the clinic by this time. Less commonly, some molecules can fit inti the major groove; the classic example is another oligonucleotide strand which if complementary to the sequence can wrap around in the major groove forming a triple stranded DNA structure.
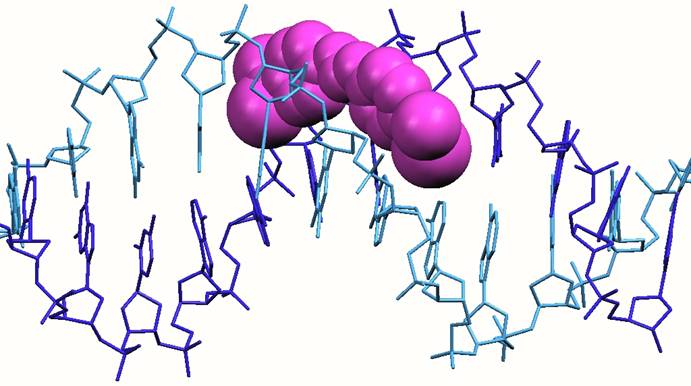
Structure of a DNA oligonucleotide with a berenil drug (shown in pink) bound in the minor groove
DNA alkylation or metallation This mechanism of DNA binding involves forming direct bonds to the DNA bases that hold the genetic information and causing distortions to DNA structure. This mode of action is used by the platinum drugs that are among the most widely used anti-cancer agents in the clinic today. The original platinum drug cis-platin (marketed as ‘platinol’) was discovered in the mid 1960s when its mode of DNA-binding was first established. It was introduced into the clinic in the 1970s. Three further platinum drugs (carboplatin – trade name ‘Paraplatin’ -, nedaplatin and most recently oxaliplatin – trade name Eloxatin) are also in clinical use. Clinicians refer to this general group of chemotherapy drugs as alkylating agents and the group also include the so-called ‘mustards’ which add alkyl groups to the bases.

Structure of a DNA oligonucleotide withcis-platin bound to two adjacent G bases
In addition to these modes of binding it is possible for species (such as alkali metal cations) to interact with the sugar phosphate backbone although this is a less useful interaction in drug design.
For the last 40 years these modes of DNA binding have dominated thinking in the field. Although they have been extended to allow recognition of other DNA structures such as triplex and tetraplex DNA the design principles have remained the same and DNA recognition drugs that have have used only these three ways to recognise the DNA. Now the Birmingham and Barcelona teams have found a fourth which is completely different and opens up entirely new possibilities for drug design.
The scientists have developed a synthetic drug agent that targets and binds to the centre of a 3-way junction in the DNA. These 3-way junction structures are formed where three double-helical regions join together. They are particularly exciting as they have been found to be present in diseases, such as some Huntington’s disease and myotonic dystrophy, in viruses and whenever DNA replicates itself (the replication fork), for example, during cancer growth.
First of all, the Birmingham team created a nanosize synthetic drug in the shape of a twisted cylinder. Together with researchers in the UK, Spain and Norway they showed that is had unprecedented effects on DNA. Now molecular level pictures taken by the Barcelona team have shown that it binds itself in a new way to the DNA, by fixing itself to the centre of a DNA junction, which had three strands. It is all held together because the cylinder is positively charged and the DNA is negatively charged. In addition the drug is a perfect fit in the heart of the junction: a twisted triangular peg in a twisted triangular hole.
There are several non-covalent interactions which appear important in the binding of the drug within the heart of the 3-way junction
Electrostatic interactions. The cylinder has a +4 charge. The DNA is negatively charged. These charges attract each other.
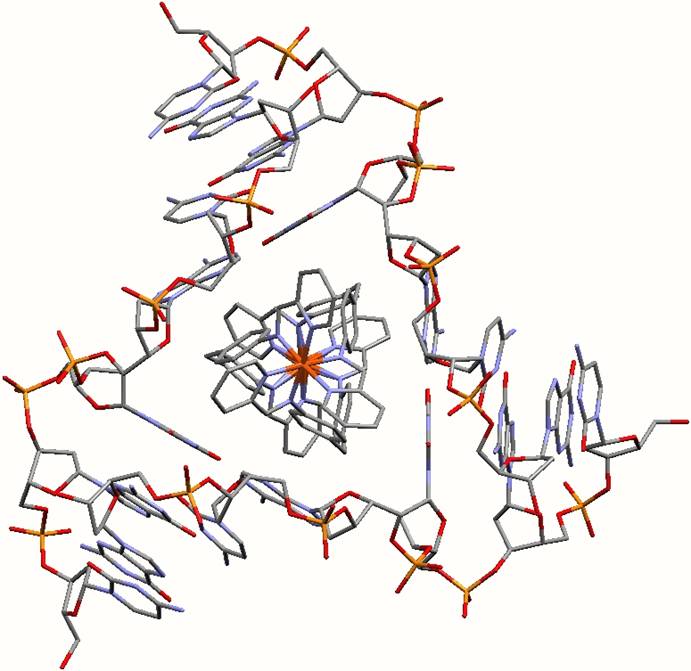
The tetracationic cylinder sits in the heart of the junction formed by the anionic DNA strands
Face-face pi- stacking interactions. Planar aromatic rings are frequently stacked upon each other. It is now recognised that this is an energetically favourable process and has been used in a variety of non-covalent molecular designs. The cylinder has 6 phenyl rings placed about its centre. These 6 phenyl rings form almost perfect pi-stacking interactions with the 6 DNA bases that are placed at the junction as the 3 double-stranded arms come together. The two phenyl rings from a strand of the cylinder stack with the two bases of one of the strands of the DNA. This is shown in the image below. This stacking is almost too good to believe (!) and leads to the cylinder fitting into the junction just like a hand into a glove.
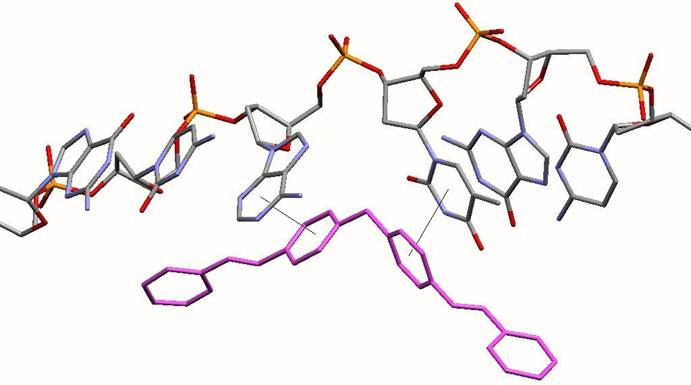
Diagram illustrating how each individual DNA strand stacks on the phenyl rings of the strands of the cylinder (pink) at the junction point. The rest of the DNA and cylinder have been excluded for clarity
Sandwiching interactions in the minor grooves. One end of the cylinder protrudes into the region where the three minor grooves of the DNA join together. The three pyridine rings at this end of the cylinder lie down the three minor grooves and are fit snugly as is seen in other minor groove binding drugs
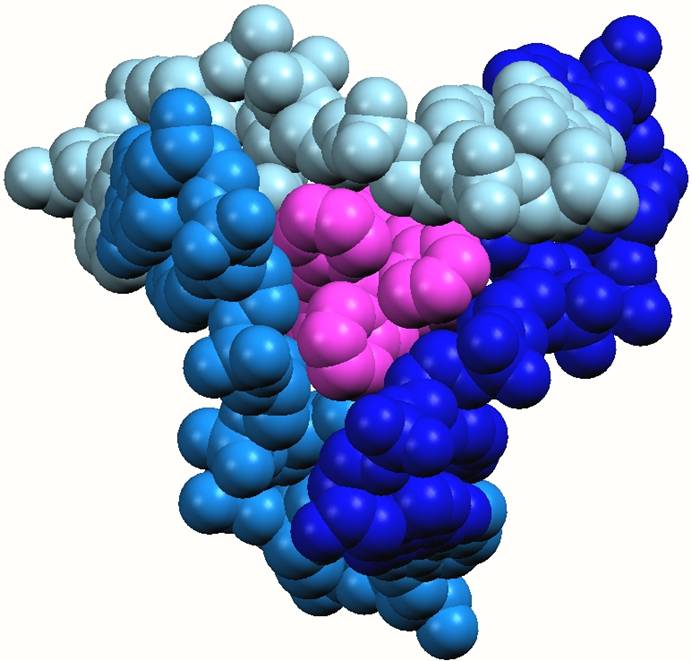
The cylinder (pink) in the heart of the junction viewed from the minor groove side and emphasising the sandwiching of the three pyridine rings of the cylinder in the three minor groove
CH…N Hydrogen bonding The outer surface of the drug is a hydrocarbon framework. The most acidic (delta positive!) of the protons is expected to be the hydrogen attached to the C=N-M unit. Consistent with this, the imine hydrogen adjacent to the minor groove end of the cylinder forms a CH…X hydrogen bond with the adenine ring (N3) at the junction.
Hydrophobic effects. Although the cylinder caries a tetracationic charge, the outer surface is comprised of hydrocarbons. It will therefore be a hydrophobic surface that will favour residence in the hydrophobic pocket formed by the DNA bases at the junction.
The type of DNA used to get crystals was a palindromic DNA hexanucleotide. Palindromic DNA is a sequence which is self-complementary (can recognize itself). Usually such DNA gives a double-stranded (ds) B-DNA structure. However, for a palindromic sequence, full Watson–Crick hydrogen bonding can, in principle, be satisfied within any oligomeric formulation (ds-DNA, three-way junction, four-way junction etc.). Entropic considerations usually would dictate adoption of a ds-structure from the library of possibilities. In this instance the triple-helical drug molecule has selected structure (the three-way junction) to which it binds most effectively, therefore driving the equilibrium exclusively to this structure.
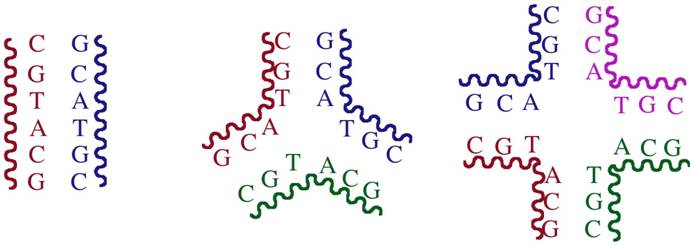
Schematic representation of how palindromic DNA can form a double-stranded DNA, a 3-way junction or a 4-way junction
The structures of the drug in the presence and absence of the junction are essentially identical confirming that the drug structure is unperturbed on binding.

A structure of a 3-way junction in complex with a protein (Cre recombinase) has previously been reported. The structure of the DNA in that structure is almost superimposable with that of the DNA in this junction.
These two observations confirm that the cylinder is recognising the 3-way junction in its biologically relevant form (and not an unusual or deformed 3-way junction). They also confirm the almost perfect fit between the cylinder and the junction.
A number of current anti-cancer drugs target disease at DNA level, but they are not specific in their approach. This is one of the reasons that they can cause unpleasant side effects. A goal has therefore been to design drugs that bind only to a very specific sequence of the DNA. This is a great challenge due to the number of bases that must be recognised to achieve effective sequence selectivity. Recognition of a specific DNA structure is a very attractive alternative to that approach.
Moreover many of these drugs suffer from developed resistance as the body learns how to deal with drugs that act in a particular way. By creating drugs which act in completely different ways this acquired resistance could be overcome.
The work is part of a trans-European collaborative effort led by Prof. Mike Hannon, leader of the Birmingham research team and based around the agents developed by that team. The consortium which is funded by the European Commission Framework research programme involves research teams at: CSIC Barcelona, Spain (Miquel Coll and co-workers); Chalmers University, Gothenburg, Sweden (Bengt Norden, Per Lincoln and co-workers); Bergen University, Norway (Einar Sletten and co-workers), University of Barcelona, Spain (Virtudes Moreno, Anna Grandas and co-workers); Institute of Biophysics, Brno, Czech Republic (Victor Brabec and co-workers). In addition there is a more local collaboration with the group of Alison Rodger at the University of Warwick. The work of the EU consortium is focused on designing and studying the DNA binding of synthetic agents that are similar in size to the agents biology uses to recognise DNA.