|
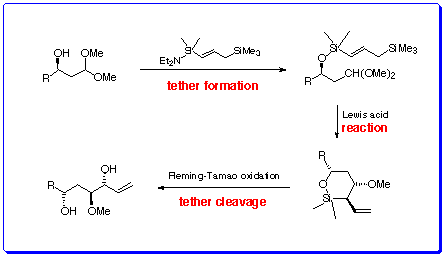
Using the Temporary Connection in Acyclic StereocontrolThe advantages of conducting intra- over intermolecular transformations are manifold: by connecting two reactants through a suitable tether, the resulting intramolecularisation imposes additional conformational constraints, restricting the number of defined transition states and leading to increased levels of regio- and stereoselectivity in the end-products.Intramolecular allylation has been shown to be an efficient method for C-C bond formation.In most cases the allyl metal species has been connected to the electrophile through a simple extension of the carbon chain with carbocycles being the end-products.The use of a more temporary connection between the allyl reagent and electrophile partner would not only exploit the advantages associated with intramolecular processes, but with the possibility for subsequent cleavage of the temporary tether, would also provide a means for remote acyclic stereocontrol, which remains a challenging area in modern organic synthesis.We are interested in preparing allylsilanes which bear additional functionality suitable for temporarilytethering the reagent to a variety of electrophilic substrates via a group proximal to the reacting centre.
The site of attachment provides a source of asymmetry from which chiral information can be transmitted during the formation of new stereogenic centres along the chain. Treatment with a Lewis acid then effects cyclisation providing highly functionalised products in a stereoselective manner, ripe for further elaboration by manipulating the silyl tether. We are particularly interested in applying this methodology to the stereoselective synthesis of C-glycosides and higher order sugars:
Project SummaryBack to Research Topics |
|
University Of Birmingham I School of Chemistry I Getting to the Department |