|
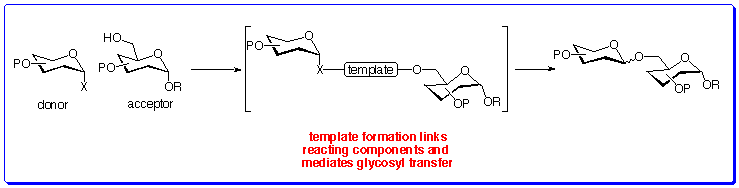
A Biomimetic Mechanistic Approach To Stereoselective GlycosylationThe last decade has witnessed a renaissance in oligosaccharide chemistry, fuelled by the increasing realisation that oligosaccharides play a crucial role in a host of biological processes, ranging from viral and bacterial adhesion to cells, protein trafficking, and intercellular recognition. Access to oligosaccharides from natural sources is possible, although usually only in small quantities, which do not meet current demand.One method for providing larger quantities of these important molecules is through chemical synthesis. Stereoselective glycosylation is central to the efficient synthesis of oligosaccharides but remains a difficult process. The majority of synthetic glycosylation methods are intermolecular processes and are consequently afflicted with many problems.While there are many methods for addressing these problems, these are not general.We are interested in using a template to temporarily link the reacting sugar partners, and in doing so, activate both the donor and acceptor. The potential intramolecularity of the reaction and preorganised nature imparted by an appropriate linker render the role of the tether analogous to that of glycosyl transferases in natureís glycosylation reactions.
Back to Research Topics |
|
University Of Birmingham I School of Chemistry I Getting to the Department |