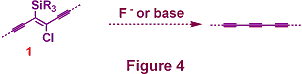|
|
|
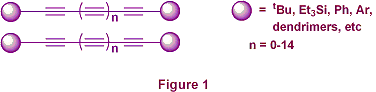
b-(Chloro)vinylsilanes - Application to the Synthesis of Masked TriynesThe shape and reactivity of the alkyne functional group, particularly when it is incorporated into highly p-conjugated systems, can lead to processing and handling problems. For example, self-condensation of oligoynes, leading to intractable polymeric mixtures, can occur as a consequence of short intermolecular distances, especially in the solid state. Methods that stabilise such conjugated frameworks are therefore particularly desirable. Several groups have reported the synthesis of systems where the rod-like oligoyne chains are kept at a distance from one another by incorporating bulky end-groups (Figure 1).[1] The major disadvantage of this approach, however, is that the stabilising effect of the terminal groups drastically decreases with increasing chain length.
An intriguing alternative approach has been recently reported by Gladysz where protection of the sensitive oligoyne chain was achieved by means of two sp3-carbon chains twisting in a double-helical motif around the conjugated framework (Figure 2).[2]
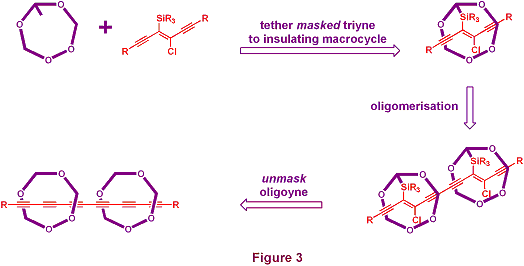
We wish to investigate an alternative approach, specifically to exploit rotaxane chemistry to encapsulate an oligoyne chain. The concept is illustrated in cartoon form in Figure 3. We propose to do this by temporarily masking a fragment of the oligoyne, and tethering this to an insulating macrocycle, only releasing the oligoyne chain at a later stage in the synthesis, when it will be protected from the external environment by the encapsulating macrocycle.
We are interested in exploiting b-(chloro)vinylsilanes as masked forms of alkynes; exposure to fluoride or base effects dechlorosilylation and provides a mild and selective route to the corresponding alkyne (Figure 4). The success of this approach calls for an efficient method for the synthesis of the generic masked triyne 1.
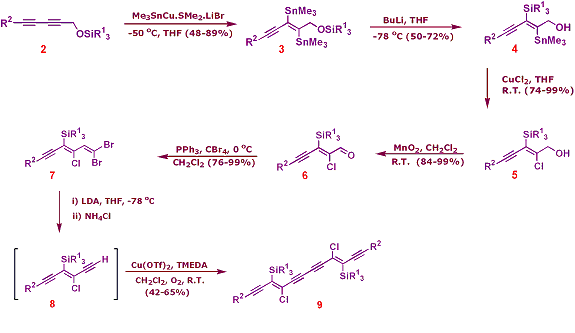
Zweifel and Leong have reported that unsymmetrically substituted buta-1,3-diynes react with the stannylcopper species, Me3SnCu.SMe2.LiBr, in a highly regio- and stereoselective fashion to afford (E)-bis-stannane products.[3] They went on to show that the internal stannyl substituent could be selectively lithiated and trapped with simple electrophiles.[3] We envisaged that we might be able to apply this methodology, which had previously been demonstrated on only two simple substrates, to a system that would allow subsequent elaboration into our target molecule 1. Our synthetic strategy is outlined in below.[4]
Bis-stannylation of the unsymmetrically aryl- and silyl-substituted buta-1,3-diyne 2 provides bis-stannane 3 with excellent stereo- and regioselectivity. Selective monolithiation at the internal site then generates a nucleophilic vinyllithium species which is set up to undergo a 1,4-retro-Brook rearrangement, thus installing the vinyl silane 4 in a regioselective fashion, and at the same time freeing up the alcohol for subsequent elaboration into the second triple bond. Stereospecific chlorodestannylation of the remaining tin residue with CuCl2 then installs the vinyl chloride 5. Allylic oxidation to the aldehyde 6 and subsequent dibromoolefination affords the dibromoolefin 7. This undergoes a Fritsch-Buttenberg-Wiechell rearrangement on exposure to an excess of LDA, providing the target acetylene 8. Due to its instability, especially in the solid state, this is used directly in the subsequent dimerisation. Thus, treatment of 8 in a modified Glaser-Hay oxidative coupling affords the dimer 9 in good yields over the two stages. The use of copper(II) triflate with its non-nucleophilic counter-ion was crucial to avoid degradation of the product.
Finally, chlorodesilylation of dimer 9a, where the end-caps are bulky triisopropylsilyl groups, is performed using potassium carbonate in methanol / THF affording the stable hexamer 10a, hence, demonstrating our ability to unveil the masked oligoyne using very mild conditions (Scheme 2).
References:[1] (a) T. Gibtner, F. Hampel, J.-P. Gisselbrecht and A. Hirsch, Chem. Eur. J.,2002, 8, 408-432; (b) R. Eastmond, T. R. Johnson and D.R.M. Walton, Tetrahedron Lett., 1972, 28, 4601-4615. [2] J. Stahl, J.C. Bohling, E.B. Bauer, T.B. Peters, W. Mohr, J.M. Martin-Alvarez, F. Hampel and J.A. Gladysz, Angew. Chem. Int. Ed., 2002, 41, 1871-1876. [3] G. Zweifel and W. Leong, J. Am. Chem. Soc., 1987, 109, 6409-6412. [4] S.M.E. Simpkins, B.M. Kariuki, C.S. Aricó and L.R. Cox, Org. Lett., 2003, 5, 3971-3974. Back to Research Topics |
|
University Of Birmingham I School of Chemistry I Getting to the Department |